Patient Support Services will work with your patients to assess eligibility for
financial assistance

Co-pay Assistance
Eligible commercially insured patients may pay as little as $0 for INBRIJA*

Patient Assistance Program
If patients cannot afford their INBRIJA prescription, they should call 1-833-INBRIJA and we may be able to help
* |
Please note, restrictions and maximum benefits apply to Merz's Co-Pay Assistance Program, including monthly and yearly maximums. For more information, call 1-833-462-7452. Merz may modify these maximums or discontinue the program at any time. In this case, any change to what commercial patients are required to pay will be communicated to patients by their specialty pharmacy. The actual amount patients have to pay will vary depending upon these maximums and their insurance benefit. See Full Terms and Conditions. |

Patients have access to INBRIJA through most commercial health plans22
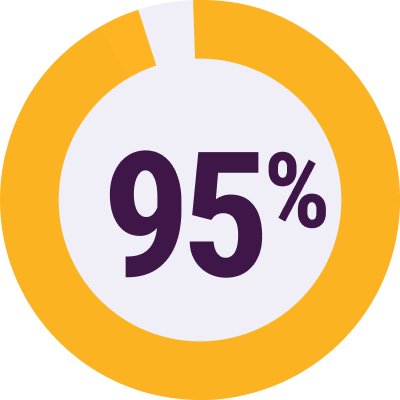
of commercial lives
covered or better
Data on file as of February 2, 2024.
Coverage varies by payor
For patients who may need additional support please contact the HUB at 1-833-INBRIJA

Medicare Part D changes may make INBRIJA more affordable for patients

Medicare Part D Changes
New Medicare Part D out-of-pocket costs are capped at $2,100—a key yearly benefit for INBRIJA patients

Medicare Extra Help Program
If you are enrolled in Medicare and have limited financial resources, you may qualify for the Medicare Extra Help program to help pay for some healthcare and prescription drug costs. Ask us for details about the program
The Medicare Extra Help program is a federal program and is not managed by Merz

Medicare Part D Flashcard
Affordability update: see how your INBRIJA patients can take advantage of the NEW Medicare Part D savings features
Terms and conditions
*Co-pay program eligibility, Terms and Conditions, and Program Limitations.
Patients must:
- Be 18 years of age or older
- Be prescribed INBRIJA for an FDA-approved indication
- Have commercial health insurance
- Not be covered by any federal, state, or government-funded healthcare program, such as Medicare, Medicare Advantage, Medicare Part D, Veterans Affairs, Department of Defense, or TRICARE
This offer is valid only in the United States, excluding where its otherwise prohibited by law.
Patients who move from commercial to federally funded or state funded insurance will no longer be eligible for the program. Proof required for receiving payment for out-of-pocket drug costs includes a valid explanation of benefits (EOB) or specialty pharmacy invoice, which must be submitted within 180 days after each treatment. Patient/Guardian may not and agrees not to seek reimbursement for value received from the Program from any third-party payers, including flexible spending accounts or healthcare savings accounts. If at any time patient begins receiving coverage under any federal, state, or government-funded healthcare program, Patient is no longer eligible to participate in the Program and must call 1-833 INBRIJA (1-833-462-7452) between 8 AM - 8 PM ET to stop participation. Restrictions may apply. This is not health insurance.
Patient/Guardian and pharmacist are responsible for notifying insurance carriers or any other third party who pays for or reimburses any part of the prescription filled using the Program as may be required by the insurance carrier's terms and conditions and applicable law
Once a patient is successfully enrolled into the Program, they will be automatically re-enrolled annually, for as long as your patient remains eligible. The patient is obligated to notify the program within thirty (30) days of any change in information provided in patient's enrollment form. The patient may notify the Program at any time to terminate participation in the Program. The patient may submit a new enrollment form to make changes to any Program elections. This offer may not be combined with any other coupon, discount, prescription savings card, free trial, or other offer for INBRIJA.
This is a limited time offer, and Merz reserves the right to rescind, revoke, amend or terminate this offer, or the program in its entirety, at any time without notice
Patient Assistance Program
For information about the eligibility requirements of the Merz Patient Assistance Program, call 1-833-INBRIJA (1-833-462-7452).