Patient Global Impression of Change
Based on the prespecified statistical hierarchy in the pivotal phase 3 clinical trial, patient global impression of change (PGIC) and OFF time were not eligible to be evaluated for statistical significance; however, analysis was conducted and nominal P values are provided for descriptive purposes, but conclusions cannot be drawn.1,21
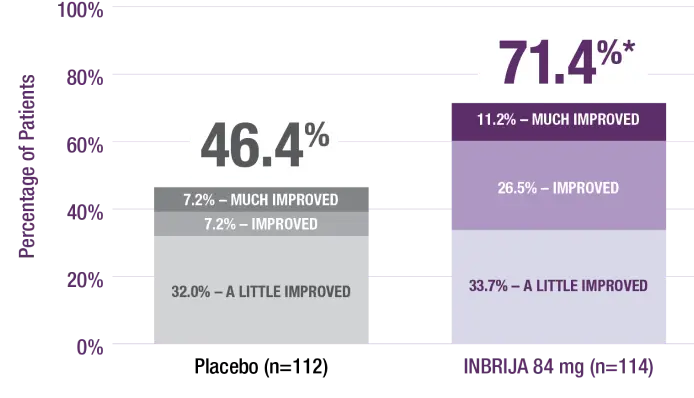
More INBRIJA patients reported improvement in PGIC compared with placebo at week 121,22
* |
P<0.001; the nominal P value was not statistically significant per the prespecified hierarchy.1 |
|
Percentage of responses to survey question: “How has the addition of study drug changed your Parkinson’s disease?”22 |