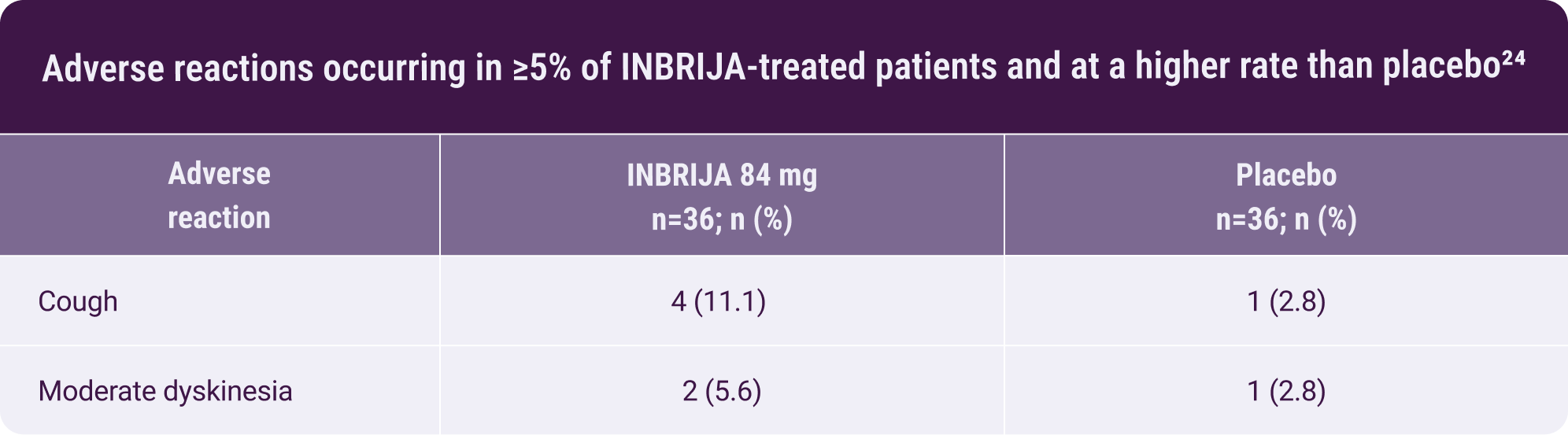
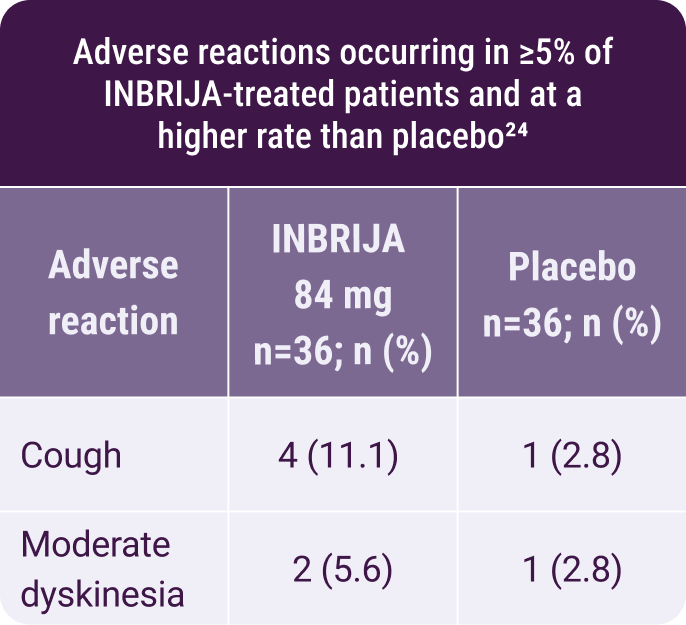
- There were no discontinuations due to adverse reactions24
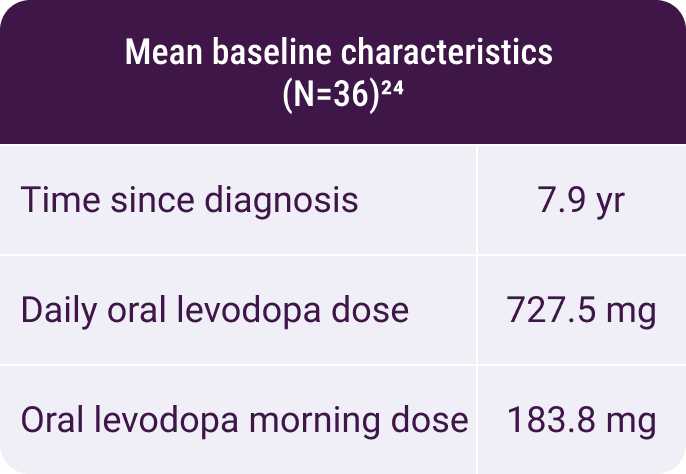
In a randomized, placebo-controlled study using INBRIJA during
morning OFF episodes24:
Moderate dyskinesia was reported in
2 of 36 patients treated with INBRIJA
No additional safety
concerns
were reported
CD/LD, carbidopa/levodopa.