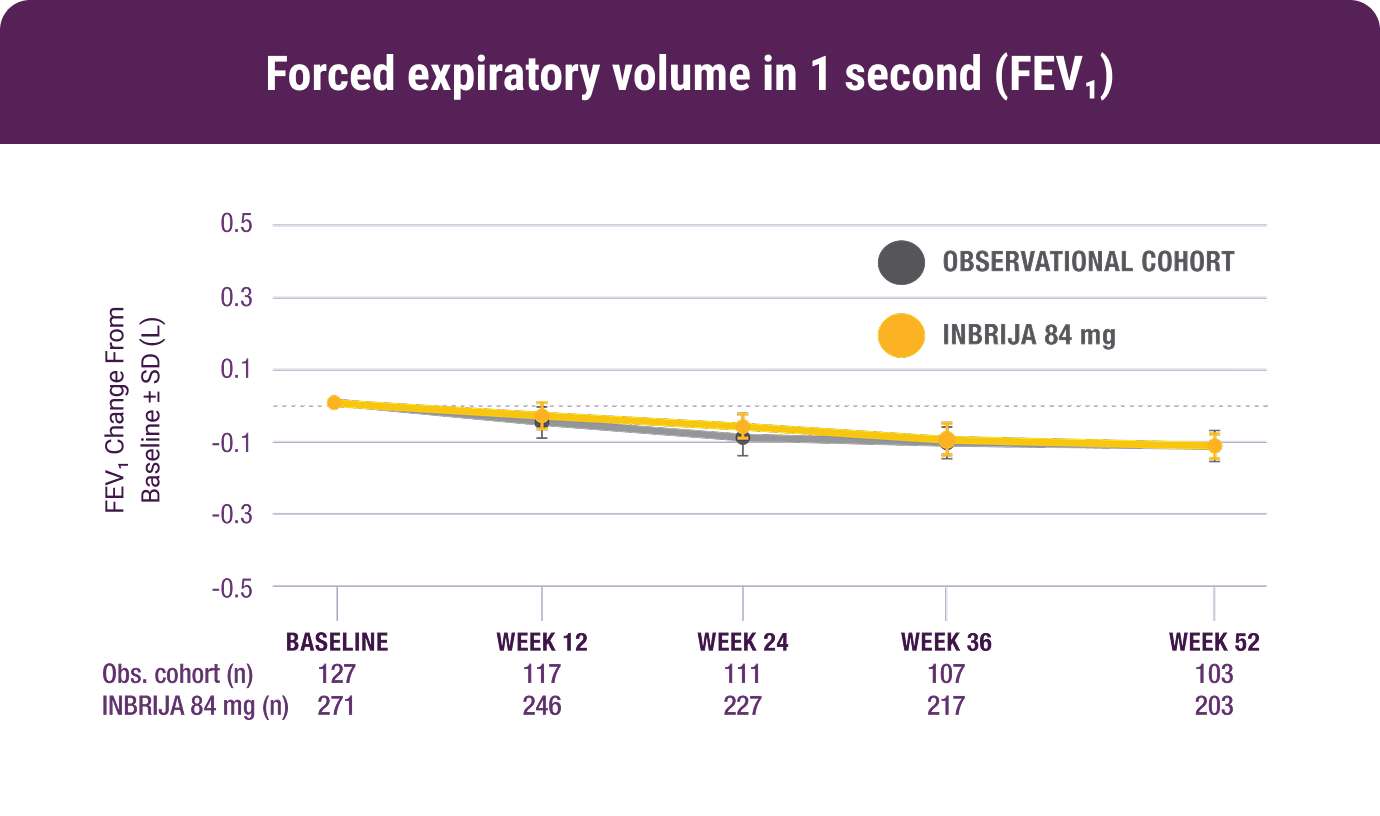
The most common adverse reactions (≥5% of INBRIJA‑treated patients and at a higher rate than observational cohort) were cough (13.3% vs 0.8%), fall (8.1% vs 5.5%), nasopharyngitis (6.6% vs 5.5%), and dyskinesia (6.3% vs 3.9%)
The most common adverse reaction leading to discontinuation was cough in 3 patients in the INBRIJA cohort
CD/LD, carbidopa/levodopa; COPD, chronic obstructive pulmonary disease; PD, Parkinson's disease.