INBRIJA starts to work in as early as 10 minutes so patients can get back in charge of their day
UPDRS Part III score change from 0 to
60 minutes post dose at week 121,*

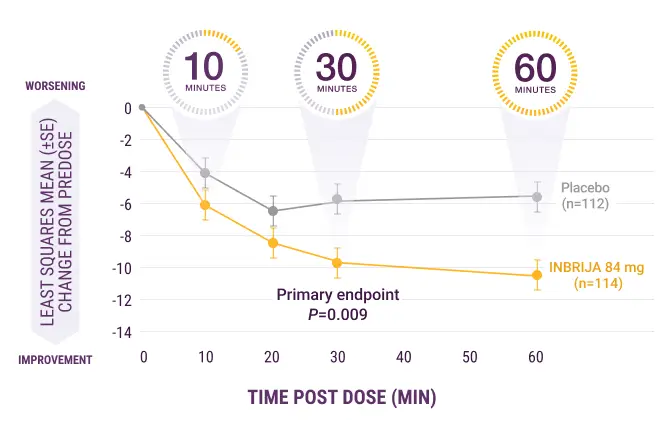
INBRIJA maintained efficacy up to 60 minutes post dose1
Significantly more patients returned to an ON state and sustained that ON state through 60 minutes post dose when taking INBRIJA 84 mg compared with placebo (58% vs 36%; P=0.003)1,†


Can be taken anytime, anywhere,*
INBRIJA is the only inhaled levodopa
that can be used to treat OFF
episodes right when they happen21
first pass metabolism3
experiencing OFF episodes21
baseline PD treatment modifications3
* |
Treatment with INBRIJA may cause drowsiness, sleepiness, and sudden sleep attacks while engaging in activities of daily living; avoid driving and operating heavy machinery while taking this medication.21 |
† |
Based on a subjective assessment by study investigators of those subjects who turned ON within 60 minutes of INBRIJA (or placebo) administration and then remained ON at 60 minutes post dose. |
|
LD, levodopa; PD, Parkinson’s disease; SE, standard error; UPDRS, Unified Parkinson's Disease Rating Scale. |
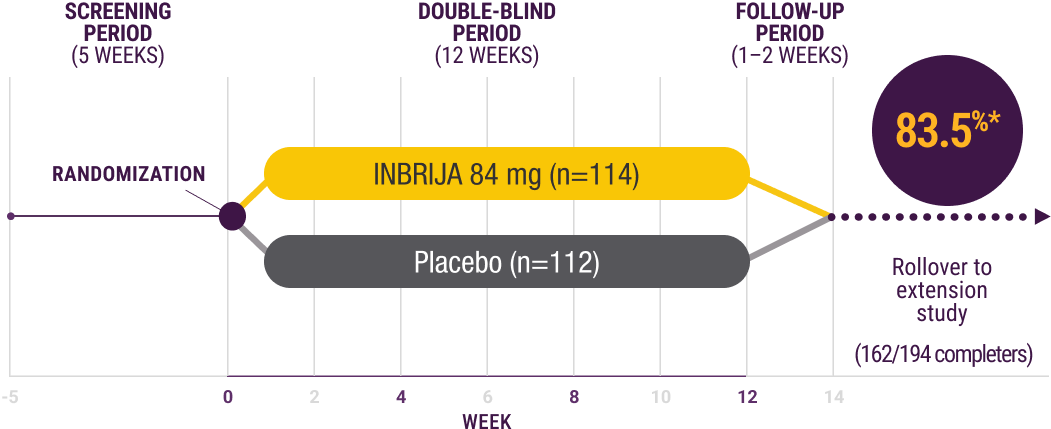
INBRIJA pivotal study design
SPAN-PD was a 12-week, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of INBRIJA for the treatment of OFF episodes in patients with PD1
Study design (n=226)1
Used at home:
- Used at the start of an OFF episode1
- Continued use of usual PD medications, including CD/LD21
- Self-administered as needed, no more than 5 times during the waking day4
In-clinic dosing at weeks 0, 4, 8, and 12:
- Patients took morning CD/LD as usual and arrived in the ON state1
- Patients remained in clinic until they transitioned into an OFF episode22
- Patients self-administered study drug at the start of the OFF episode1
UPDRS PART III† motor score at 30 minutes1
Select baseline criteria for the SPAN-PD study
* |
About ⅓ of patients in the extension study were in the placebo arm and ⅔ of patients were in the INBRIJA arm.22 |
† |
Not mean value. |
‡ |
Includes early morning OFF. |
|
CD/LD, carbidopa/levodopa; COMT, catechol-O-methyltransferase; COPD, chronic obstructive pulmonary disease; MAO-B, monoamine oxidase B; PD, Parkinson’s disease; PGIC, patient global impression of change; UPDRS, Unified Parkinson's Disease Rating Scale. |
Safety Data from the Pivotal Study
INBRIJA has a well-established safety profile
Most common adverse events occurring in ≥5% of
INBRIJA-treated patients and at a higher rate than placebo1
INBRIJA 84 mg (n=114) |
Placebo (n=112) |
|
|---|---|---|
| Cough | 15% | 2% |
| Upper respiratory tract infection |
6% | 3% |
| Nausea | 5% | 3% |
| Discolored sputum | 5% | 0% |
* |
Sensation of choking was identified during postapproval use of INBRIJA. |